IPSS Research
IPSS Research
International Pediatric Stroke Study (IPSS)

The International Pediatric Stroke Study (IPSS) is the world’s largest and most successful childhood stroke registry and network made up of dedicated clinicians, scientists and research staff from over 100 institutions in 34 countries. They have prospectively enrolled over 8,000 patients in an effort to conduct high-quality clinical and imaging research studies. The IPSS will continue to conduct transformational research with help and support from partner organization IPSO. Together, the IPSS and IPSO will provide the foundation for scientific and clinical collaborations, as well as advocate for the paediatric stroke community worldwide.
IPSO members are welcome to get involved with the IPSS. Learn more below!
History
In 2003, Dr. Gabrielle DeVeber at the Hospital for Sick Children, with 11 co-Investigators and a multi-center clinical research grant from the Child Neurology Society/Child Neurology Foundation, established the International Pediatric Stroke Study (IPSS), the world’s first pediatric stroke registry. During the initial 4 study years, 30 participating IPSS centers enrolled 1,187 neonates and children and published the first 8 IPSS papers informing pediatric stroke practice worldwide. With additional funding support from the National Institute of Neurological Disorders and Stroke (NINDS), philanthropic donors (The Auxilium Foundation), and other source funding for targeted studies on stroke classification (Ped-NIHSS, CASCADE), etiology (the Vascular Effects of Infection in Stroke, VIPS), thrombolytic safety (Thrombosis in Pediatric Stroke, TIPS), and seizures (Seizures in Pediatric Stroke, SIPS), the IPSS has expanded globally and recruited additional pediatric stroke experts from multiple disciplines in an effort to better understand, prevent, and improve outcomes in pediatric stroke.
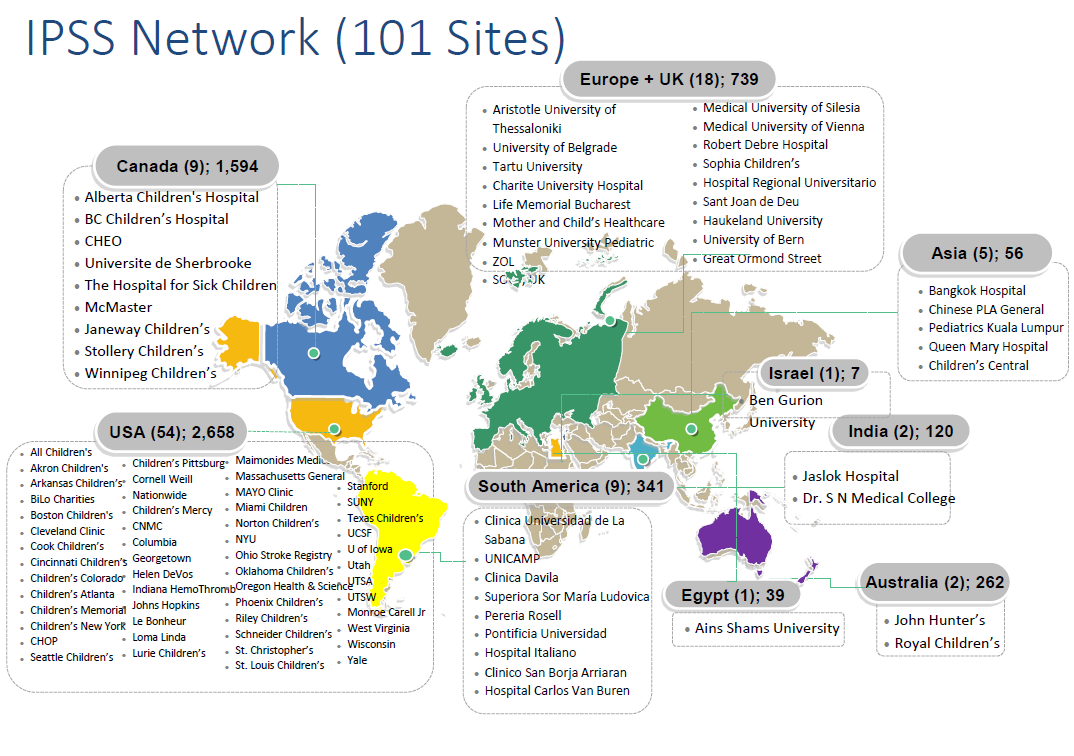
The IPSS has since continued to serve as a highly successful study vehicle facilitating data entry from over 150 Investigators and Research Staff at 100 institutions across 34 countries worldwide. To date (2024), the IPSS Investigators have enrolled over 8,000 patients with ischemic stroke and hemorrhagic stroke (including those at high risk of stroke) and have published over 40 papers. The registry for hemorrhagic stroke and vascular malformations started more recently- read more about that effort here.
IPSS Network
Mission & Objectives
The missions of the IPSS are:
Perform international collaborative research aimed at understanding, preventing, and improving outcomes in pediatric stroke.
Support the mission of IPSO by serving as a clinical and imaging research network with an attractive platform for sites to enroll patients into a centralized, shared registry database.
Provide IPSO and the pediatric stroke community with data that can be used to support professional and public education; families with pediatric stroke members; and the development of future research and clinical trials.
The specific objectives to promote this aim are to:
1. Ascertain in a prospective, consecutive cohort study the numbers of newborns and children with ischemic stroke, their stroke sub-types and risk factors, their current treatments and outcomes within our centers. These data will provide the rationale and feasibility data for our group to design and implement the initial randomized controlled trials (RCTs) in pediatric stroke as well as other fundable grant proposals.
2. Develop standardized data collection forms and an appropriate database with web-based data entry from multiple study sites.
3. Develop standardized guidelines for (1) diagnosis, (2) investigation of risk factors, (3) outcome assessment,(4) antithrombotic therapies for neonates and children with arterial ischemic stroke (AIS) and cerebral sinovenous thrombosis (CSVT) and (5) partner with other relevant networks caring for stroke
4. Obtain funding to support and conduct additional multi-centre IPSS sub-studies
Please note that the IPSS objectives are currently being reviewed by IPSS and IPSO leadership committees to accommodate the growing needs of the IPSS Investigators and our IPSO partnership.
Data Policy
The IPSS Publications Committee (PC) was established for the purpose of enabling timely research publications whilst ensuring the maintenance of ethical standards, the primacy of patient privacy and fair distribution of authorship for academic contributors.
The PC strives to recognize all contributing Investigators through authorship on IPSS papers. Contribution may be in the form of patient recruitment or intellect (participation in study design, meeting attendance, sub-group participation, contribution to complex stroke case etc.).
Mentorship and assistance to co-authors is provided by the Publications Committee, where needed, specifically by the PC liaison.
Junior Investigators are a priority to the committee, for whom authorship is critical for academic promotion.
Review the IPSS Publications Committee Policy for more information on how to request the use of IPSS data. If interested in submitting a request, download and complete the IPSS Research Request Form below and send to
Data Ownership
Data Use
Grant-funded IPSS Studies
IPSS and SARS-CoV-2
With reports of stroke in young adults with COVID-19 and emerging information about the novel multisystem inflammatory syndrome in children, there have been concerns that COVID-19 may be a risk factor for stroke among children. Thankfully, there seems to be strong evidence that children have less severe consequences of the novel coronavirus infection; however, the SARS virus in 2002-2003 caused some children to be hypercoagulable (increase blood clotting). As a result, the IPSS deemed it important to evaluate the role of the COVID-19 virus in pediatric ischemic stroke patients by administering an international survey (distributed in early June 2020) to capture the number of COVID-19 positive pediatric stroke cases between March 2020 and May 2020. The survey also evaluated SARS-CoV-2 (virus that causes COVID-19) testing practices for pediatric stroke patients among our international group of investigators and sites. The survey can be found here and results will be published in Annals of Neurology. The survey will be re-distributed in the Fall 2020 to evaluate the virus’ role in pediatric stroke as the pandemic continues.
Resources for IPSS Investigators
Clinical Data
Historically, IPSS clinical data was captured in Oracle (2003-2014). Since 2015, all clinical data has been captured in REDCap when the data collection form also developed to capture specific patient sub-groups and classifications of pediatric stroke.
Link to Clinical Database (REDCap): https://redcapexternal.research.sickkids.ca/
Download PDF of IPSS Eligibility Criteria
Neuroimaging Data
Please contact IPSS Central for more details.
